Wednesday, August 1, 2007
Belated (but oh so necessary) Goodbye
Well I'm really sad this is over. I realize it ended a week ago, but I've really been missing everyone these few days wandering off to lunch alone! This was probably the greatest single experience I've had. I don't think I'll ever forget any of you, or the amazing things I've learned this summer. How many other high school kids have met a Nobel Laureate and discussed and presented advanced scientific topics to and with professors, on their level? Needless to say, our scientific experiences have been inestimably inspiring. Another aspect that struck me, however, was that this summer was also amazing in that, as high school students, we learned to work and communicate on the level of adults. The speakers, mentors, and audiences that we have met never looked down on us as kids that they have to deal with for a summer. Far from it; they showed genuine enthusiasm working with us and teaching us about their respective fields. We learned as a part of the scientific community, completely incorporated rather than temporary visitors. This, I believe, is almost quite as important as the science itself. In short, I consider the Howard Hughes Precollege Program to have been nothing short of life-changing.
Tuesday, July 31, 2007
Final wrap up
Hey, great job, everyone! The poster session last Friday was a great success. It was neat to meet so many of your parents, PI's, and mentors. I'm so glad to hear that so many of you enjoyed your summer research experiences.
Thanks so much to all of those who kept posting to the blog throughout the summer. I think it was an overall success, and we'll be adding it as a requirement to next year's program.
Once again, great job everyone! Please keep us updated about your career goals and where you end up for college! Good luck this next year and enjoy the rest of the summer!
Thanks so much to all of those who kept posting to the blog throughout the summer. I think it was an overall success, and we'll be adding it as a requirement to next year's program.
Once again, great job everyone! Please keep us updated about your career goals and where you end up for college! Good luck this next year and enjoy the rest of the summer!
Thursday, July 26, 2007
Going out with a bang!
I can't believe its already the very last day working in the labs. It'll be sad to leave the lab but I'm excited for the poster session tomorrow!
Today was transplant day for one of the two patients waiting right now--very exciting!!
I was able to observe in the operating room with Dr. Markert, Julie (who works in the lab) and two other students (Dave and Ashley). After we carried over all the slices of thymus in a plastic bin and suited up in bunny-suits, hair and shoe covers, and masks we entered the operating room. A resident was actually performing the surgery with one or two doctors and several nurses, and us. It was a pretty full house but luckily we were in a pretty big room. They had started to put the central line into the baby when we arrived and it took them a while to get it positioned correctly. They start by putting a needle near the neck and then using it to guide a wire through a vein all the way to the heart, and then thread a tube over the wire and pull the wire out. They used x-rays to watch the position on the wire which we could watch on a screen. The central line will be used to give the patient calcium and nutrients while he's waiting for stomach surgery. They then started the transplant procedure by making an incision on both thigh muscles. Fortunately, one of the students was able to recognize at this point that they were not meant to be a surgeon and had to go sit down right away. I went out into the hall when the person fainted. Luckily, we were already in a hospital and some nice doctor brought us a wheelchair and some food. And so...i missed most of the actual transplant surgery. I was able to go back in the operating room just in time to see the last thymus slice inserted and watched them sew it up. Needless to say, it was a very interesting day at the hospital all around and a high light of the summer!
I'm so grateful for this opportunity, i can't imagine a better way to have spent time working this summer. Everyone in the lab made it such an enjoyable and amazing experience. Thanks to everyone in the group too, it's been fun!
Today was transplant day for one of the two patients waiting right now--very exciting!!
I was able to observe in the operating room with Dr. Markert, Julie (who works in the lab) and two other students (Dave and Ashley). After we carried over all the slices of thymus in a plastic bin and suited up in bunny-suits, hair and shoe covers, and masks we entered the operating room. A resident was actually performing the surgery with one or two doctors and several nurses, and us. It was a pretty full house but luckily we were in a pretty big room. They had started to put the central line into the baby when we arrived and it took them a while to get it positioned correctly. They start by putting a needle near the neck and then using it to guide a wire through a vein all the way to the heart, and then thread a tube over the wire and pull the wire out. They used x-rays to watch the position on the wire which we could watch on a screen. The central line will be used to give the patient calcium and nutrients while he's waiting for stomach surgery. They then started the transplant procedure by making an incision on both thigh muscles. Fortunately, one of the students was able to recognize at this point that they were not meant to be a surgeon and had to go sit down right away. I went out into the hall when the person fainted. Luckily, we were already in a hospital and some nice doctor brought us a wheelchair and some food. And so...i missed most of the actual transplant surgery. I was able to go back in the operating room just in time to see the last thymus slice inserted and watched them sew it up. Needless to say, it was a very interesting day at the hospital all around and a high light of the summer!
I'm so grateful for this opportunity, i can't imagine a better way to have spent time working this summer. Everyone in the lab made it such an enjoyable and amazing experience. Thanks to everyone in the group too, it's been fun!
Wednesday, July 25, 2007
the end, oh sorrow
Oh no! Howard Hughes is coming to an end...and it coincides with the last harry potter book too! my life is going to be slightly depressing after this. i had so much fun with everyone, and yesterday's dinner and hogwarts sculpting session and outside photo-attackingness were aMaZiNg. i can't wait for friday's poster session; we should all be really proud of our work! More later, i think i'm going to try to sneak my way into a gastric fluid extraction show.
~love from linda
~love from linda
Monday, July 23, 2007
The End
Well guys, this is the end. The final chapter of our time here. It's been a great experience, and I know everybody's excited/anxious about presenting their projects. Personally, I am not quite finished with mine. But I still have one more day to tie up loose ends. I have my conclusion written, but not my results. I'm sure I'll finish it up tonight. I can't believe all that I've learned these past 6 weeks- sequencing, PCR, gels, purifications, specs, bioinformatics, statistical tests, sea urchin development, and the ridiculously high cost of biological research. Now I know what grants are for. Though I'm still not sure what I want to do with my life career wise, I think I've learned a lot about biology and lab research this summer. There is a huge difference between just reading about something and actually doing it. Thanks to everyone in my lab and to all the ladies with the Howard Hughes Program- you guys have been really great! Thanks to all the speakers and thanks to the sea urchins for sacrificing your little lives for the greater good! So long.
Results
The second to the last week was surprisingly exciting. I was thrilled to see if we could find any associations using the lab data. Dr. Allison and Melanie assisted me in using statistical approaches to analyze my data. One of the interesting things that we found was that child IQ was predicted by genotype at one VDR SNP (RS7975128, p=0.01), and maternal smoking within the child’s first 7 years of life (p=0.03). However, the interaction between the VDR SNP and maternal smoking was not significant (p=0.40). The A/G and G/G genotypes of RS7975128 were associated with lower IQ, as was the occurrence of maternal smoking. I also attended a lab meeting and a clinical meeting.
Thursday, July 19, 2007
Formatting
So this week I was supposed to work on analyzing the data I had gathered. I had hundreds of sequences available, but not all of the genes had enough individuals to be useful. I deleted all the genes that did not have seven or more individuals available. With less than 14 haplotypes, you can’t make very reliable conclusions about that particular gene. So it turned out that only ten out of twenty-seven genes were fit for analyzing. First, I had to transfer the sequencher files to MacClade, a program that helps you align the base pairs so that you aren’t starting in the middle of a codon. The MacClade program lets me see all the sequences in the gene at the same time. The SNPs show up very clearly because each base is a different color, so if there were no SNPs, the gene sequences would just look like pinstripes. So then I had to export the MacClade files as fasta files. I had to format all the sequences individually, which was time consuming, but now I am finally done and all I have to do is give Ralph, who has written programming to analyze selection and variation frequency for sequences. I was formerly using a program called DNAsp, which essentially does the thing that Ralph’s program does, but it would not read any of the data files I made on my computer. Also, Ralph’s program not only gives you the numbers, but also tells you how certain it is in this number. DNAsp just gives you the number. On Friday and Monday I’ll probably work on fixing my poster.
Sunday, July 15, 2007
Killifish Eggs and Hatchlings
Everything has been busy in the Eco-toxicology Lab getting ready for next week. We've have been dosing the fish eggs with various contaminants trying to find the LC 50 (the point at which half of the eggs are dead). When we have the LC 50 for fish from our control site, we can use that data to compare it to how fish from a contaminated site, the Elizabeth River, deal with the same amount of contamination.
This has been harder then we first expected. The first contaminant we exposed them to was Ammonium Chloride. We tried three dose responses with killifish eggs. In the first experiment, we tried doses ranging from 7.5 mg/L to 150 mg/L. The fish had no reaction and continued with development as usual. We tried another dose ranging from 250 mg/L to 1500 mg/L and once again the killifish eggs showed no response. We did a final, more extreme experiment with doses ranging from 2,500 mg/L to 10,000 mg/L. When the eggs showed no response to this as well, we came to the conclusion that the killifish are protected inside their eggs and that the ammonia does not penetrate the egg. We later did experiments in killifish hatchlings which did show a response to the ammonia. I never thought I'd be happy to say that something died, but after watching many fish eggs survive the ammonia doses, I was glad to deliver the news that hatchlings did show the response we expected to the ammonia and that we would be able to continue on with our research.
Although, this presented an obstacle in continuing with my research of whether Elizabeth River killifish have a fitness cost associated with their adaptation to PAH's. We could no longer do research in eggs because the pesticides we were exposing them to proved to be similar to the ammonia in the sense that the killifish eggs showed no response. Instead we would need to do our research with hatchlings. Killifish eggs take two weeks to hatch. A large quantity of them, 350, will be ready from hatching tomorrow but this set us back a bit in data collection. We will conduct the majority of our research on these hatchlings next week using the data that we have gathered in the last few weeks with the dose response experiments, to know what the correct doses are to reach the LC 50. It will be another busy week, ending of course with the release of the final Harry Potter book. I know many of us in the program are looking foreward to that.
This has been harder then we first expected. The first contaminant we exposed them to was Ammonium Chloride. We tried three dose responses with killifish eggs. In the first experiment, we tried doses ranging from 7.5 mg/L to 150 mg/L. The fish had no reaction and continued with development as usual. We tried another dose ranging from 250 mg/L to 1500 mg/L and once again the killifish eggs showed no response. We did a final, more extreme experiment with doses ranging from 2,500 mg/L to 10,000 mg/L. When the eggs showed no response to this as well, we came to the conclusion that the killifish are protected inside their eggs and that the ammonia does not penetrate the egg. We later did experiments in killifish hatchlings which did show a response to the ammonia. I never thought I'd be happy to say that something died, but after watching many fish eggs survive the ammonia doses, I was glad to deliver the news that hatchlings did show the response we expected to the ammonia and that we would be able to continue on with our research.
Although, this presented an obstacle in continuing with my research of whether Elizabeth River killifish have a fitness cost associated with their adaptation to PAH's. We could no longer do research in eggs because the pesticides we were exposing them to proved to be similar to the ammonia in the sense that the killifish eggs showed no response. Instead we would need to do our research with hatchlings. Killifish eggs take two weeks to hatch. A large quantity of them, 350, will be ready from hatching tomorrow but this set us back a bit in data collection. We will conduct the majority of our research on these hatchlings next week using the data that we have gathered in the last few weeks with the dose response experiments, to know what the correct doses are to reach the LC 50. It will be another busy week, ending of course with the release of the final Harry Potter book. I know many of us in the program are looking foreward to that.
BuSy Week With the ADHD samples!!!!
On Monday, I was able to plate my ADHD DNA using the Biomek robot. This made me extremely excited because I was finally able to start on my project. I left the plates to dry overnight at room temperature. The following day, I began working on my assays involving the ALAD gene and the VDR gene. I made 18 Taqman plates for 9 assays and ran 14 of those plates onto the PCR machine. I then scanned them on the 7900HT machine. Most of my TAqman went well except for two plates. Next, for Wednesday, I had to re-run the two plates that failed. I also ran 6 more plates on the PCR machine and made 2 plates for a single assay. I scanned those plates as well and they turned out great. For the rest of the day, I had to plate 20 extra plates using the Biomek robot. On Thursday, I made 5 Taqman plates in the morning. I ran them using the PCR machine and scanned them afterwards. I then added markers and detectors. Fortunately, they looked great. In the afternoon, I ran about 5 more plates and scanned them. In addition, the fire alarm went off again for the second time since I have been in CHG. On Friday, I really enjoyed the talk by Ms. Cleaver. It gave me many tips in applying for college. After that, I went to my lab and ran and scanned some plates that I had made prior. I also helped Kaia by making two agarose gels for her and running her PCR samples. Some looked good but the rest failed. Chris then helped me submit my data so that I could view them with a statistician next week. Unfortunately, two failed to submit but I’ll be able to do that on Monday. Overall, I had an exciting and this week was probably the busiest for me by far.
Sequencher
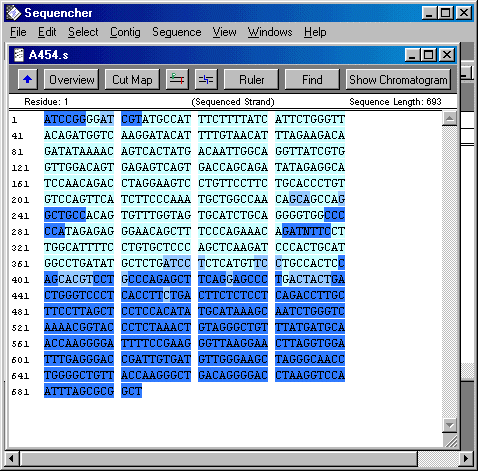
So this last week I focused on organising and cleaning up sequences in a Sequencher program. I had to find SNPs and label them by hand. Since I was reviewing 20-ish different 200 to 1000 bp sequences for 2 to 24 individuals each. THat's a lot of letters. So I didn't quite finish, but I hope to finish up on Monday. Not only did I label SNPs, I also cut off the ends of each sequence that were not reliable. Sequencher actually reads the chromatogram and translates the flourescent waves into four letters, the letters that encompass the genetic code (C, A, T, and G). Sometimes, though, Sequencher makes a bad call. When it miscalls a base or is not sure about a base, I have to fix that by changing the sequence. Once I have cleaned up all the sequences so that all sequences of the same primer pairs are the exact same length, then I will get help from some other people in the lab on analyzing the data. The picture is what the sequence looks like on sequencher. Dark blue bases mean that Sequencher is less sure about the base call, whereas light blue bases mean that Sequencher is pretty certain that the base is as it calls it.
Hi everyone!
For the last two weeks, I have been continuing to work on my Toluene experiments. Turns out that the results were not as promising as we had hoped since the E. coli conjugates do not seem to be breaking down the Toluene. :-( I have been testing different conditions to see if the toluene will degrade in more stressful environments, and some seem to be working a little better than the others. One possibility is that the original stock solution has been contaminated, since it has been sitting in the refridgerator for a while now. We should probably make a new stock solution on Monday. Hopefully Ruoting and I will be able to find advantageous conditions for the E. coli soon. :-D
Annie
Harry Potter and the Deathly Hallows
5 days. 4 hours. 27 minutes. 07 seconds.
For the last two weeks, I have been continuing to work on my Toluene experiments. Turns out that the results were not as promising as we had hoped since the E. coli conjugates do not seem to be breaking down the Toluene. :-( I have been testing different conditions to see if the toluene will degrade in more stressful environments, and some seem to be working a little better than the others. One possibility is that the original stock solution has been contaminated, since it has been sitting in the refridgerator for a while now. We should probably make a new stock solution on Monday. Hopefully Ruoting and I will be able to find advantageous conditions for the E. coli soon. :-D
Annie
Harry Potter and the Deathly Hallows
5 days. 4 hours. 27 minutes. 07 seconds.
Subscribe to:
Posts (Atom)